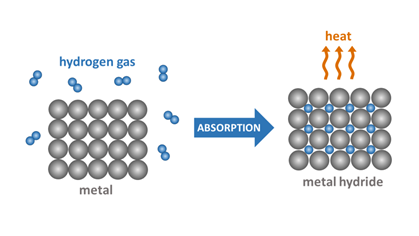
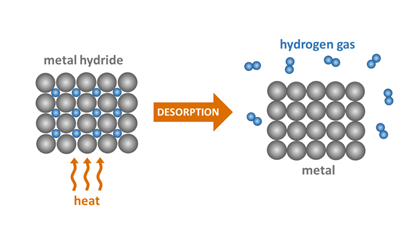
METAL HYDRIDES
Hydrogen can be stored in metal hydrides, which are compounds formed between hydrogen and a metal. The hydrogen is stored within the metal lattice and can be released through a process called dehydrogenation, which involves heating the metal hydride to a high temperature. There are several types of metal hydrides that can be used for hydrogen storage, including intermetallic compounds, complex metal hydrides, and simple metal hydrides.
Intermetallic compounds are formed between two or more metals and can store a large amount of hydrogen in their lattice. They are generally stable and can be used for long-term hydrogen storage. However, they often require high pressures or high temperatures to release the hydrogen, which can be challenging to achieve in practical applications.
Complex metal hydrides are formed between a metal and a non-metal and can store a moderate amount of hydrogen in their lattice. They tend to release the hydrogen at lower temperatures than intermetallic compounds, but they are not as stable and may decompose over time.
Simple metal hydrides, such as magnesium hydride, can store a small amount of hydrogen in their lattice and release it at low temperatures. However, they are not as stable as intermetallic compounds and tend to decompose over time.
Overall, metal hydrides are a promising option for hydrogen storage due to their high hydrogen storage capacity and the ability to release the hydrogen at relatively low temperatures. However, further research is needed to improve their stability and reduce the cost of producing them.