M4 Hydrogen storage and transport
Topic outline
-
-
Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Education and Culture Executive Agency (EACEA). Neither the European Union nor EACEA can be held responsible for them.
-
-
-
Hydrogen can be stored in a variety of ways, including as a gas, a liquid, or in a solid form such as a metal hydride or chemical compound. The most common method of hydrogen storage is as a compressed gas in high-pressure tanks. This method has the advantage of being relatively simple and inexpensive, but the storage density is relatively low, so large tanks are required to store a usable amount of hydrogen.
Hydrogen can also be stored as a liquid in cryogenic tanks, which are tanks that are designed to store materials at extremely low temperatures. Liquid hydrogen has a much higher storage density than gaseous hydrogen, so it can be stored in smaller tanks. However, the tanks and equipment needed to store and handle liquid hydrogen are more expensive and complex than those needed for compressed gas storage.
Another method of hydrogen storage is to store it in a solid form, such as in a metal hydride or chemical compound. This method has the advantage of being able to store large amounts of hydrogen in a small space, but it is generally more expensive and less efficient than the other methods.
There are also several methods for transporting hydrogen, including by truck, train, ship, and pipeline. The most common method is by truck, using tanks that are similar to those used for storing hydrogen. Hydrogen can also be transported by train or ship in cryogenic tanks, or it can be transported through pipelines like natural gas.
Overall, the development of effective and efficient methods for storing and transporting hydrogen is an important area of research and development, as hydrogen has the potential to be a way of storing energy.
-
-
-
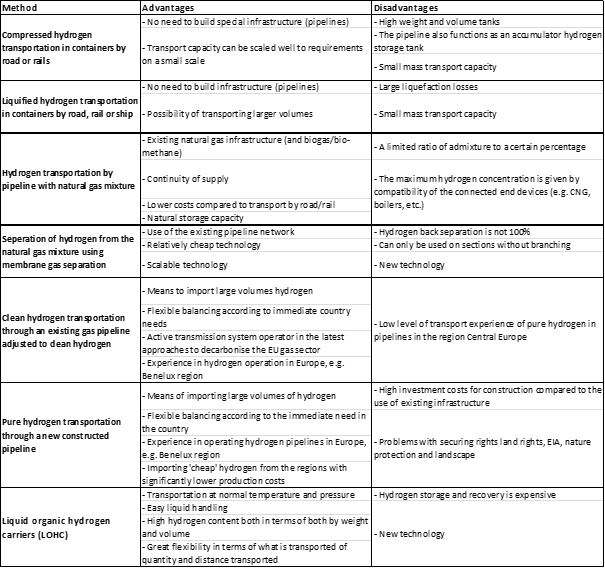
Hydrogen can be transported in several ways, including by truck, ship, rail and pipeline.
One common method of transporting hydrogen is by truck, using cryogenic liquid tanker trailers. These trailers are designed to carry liquified hydrogen at extremely low temperatures (-253°C), which reduces the volume of the hydrogen and allows for more efficient transport.
Another option is to transport hydrogen by ship, either as a cryogenic liquid or as a compressed gas. Cryogenic liquid hydrogen can be shipped in specialized tankers, similar to those used for transporting liquified natural gas (LNG). Compressed hydrogen gas can also be shipped in containers that are designed to withstand high pressures.
Finally, hydrogen can also be transported through pipelines, just like natural gas. This method is generally only practical over short distances, as hydrogen tends to leak out of pipelines more easily than natural gas. However, it can be an efficient option for moving hydrogen from a production site to a nearby storage or distribution center.
Keywords
Liquified hydrogen, compressed hydrogen pipeline, container, transport, tanker, train, truck, gas, ship, membrane separation, LOHC
-
Hydrogen can be transported in gaseous or liquid form.
Gaseous hydrogen: Gaseous hydrogen can be shipped in pressurized or refrigerated tanks on specialized ships. These tanks are designed to store hydrogen at high pressures and low temperatures to minimize the volume of the gas. Shipping gaseous hydrogen by sea is a relatively new development and is currently limited to small quantities.
Liquid hydrogen: Liquid hydrogen can be shipped in cryogenic tanks on specialized ships. These tanks are designed to store hydrogen at temperatures below -253°C and at high pressure to keep it in a liquid state. Shipping liquid hydrogen by sea is more common than shipping gaseous hydrogen, but it is still relatively limited due to the high cost of the specialized ships and handling equipment.
Hydrogen delivery refers to the transportation and distribution of hydrogen from a production or storage site to a point of use or sale. There are several methods for delivering hydrogen, including pipeline, tanker truck, rail and on-site generation.
Pipeline: Hydrogen can be transported through pipelines just like natural gas, but the infrastructure for hydrogen pipelines is currently limited.
Tanker truck: Hydrogen can be transported by tanker truck, either in its gaseous or liquid form. When hydrogen is transported in its liquid form, it must be kept at a temperature of -253°C and at high pressure to remain a liquid. This requires specialized tanker trucks and handling equipment.
Rail: Hydrogen can also be transported by rail, either in its gaseous or liquid form. However, rail transport is not commonly used for hydrogen due to the high cost of specialized railcars and handling equipment.
On-site generation: In some cases, hydrogen can be produced on-site at the point of use using natural gas, water electrolysis, or other methods. This can be a convenient option for users who need a small amount of hydrogen on a regular basis and are located near a natural gas supply.
Regardless of the delivery method, hydrogen must be handled carefully as it is highly flammable and can be dangerous if not handled properly.
Review questions
1. What are the known basic methods for hydrogen transport?
2. What is the usual pressure in containers when transporting compressed hydrogen?
3. What is the temperature of liquid hydrogen?
4. What is the typical concentration of hydrogen when transported in natural gas pipelines?
5. What is membrane separation?
6. What do you know about The European Hydrogen Backbone project?
7. Explain what the acronym LOHC means.
-
-
-
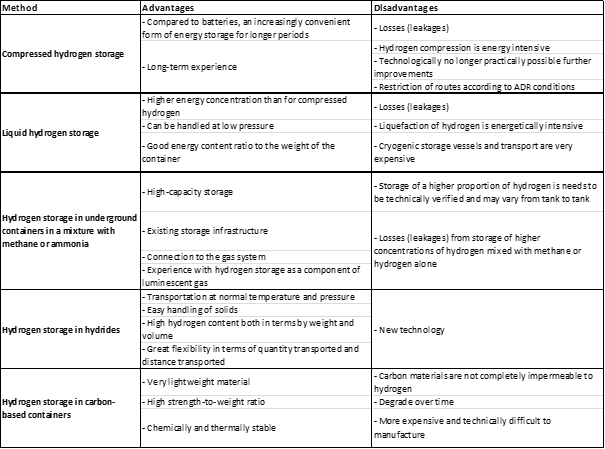
Hydrogen can be stored in various ways, including as a gas, a liquid, or in a solid form. Each method has its own advantages and disadvantages, and the most appropriate method for a particular application will depend on the specific requirements and constraints of that application.

One way to store hydrogen is as a gas, which can be done by pressurizing it in a tank or cylinder. This is a simple and relatively cheap method, but the storage density is relatively low, so a large volume is required to store a significant amount of hydrogen. Additionally, high pressure tanks can be heavy and may require special handling and safety measures.
Another way to store hydrogen is as a liquid, which can be achieved by cooling it to a temperature below its boiling point. Liquid hydrogen has a very high storage density, so it is possible to store a large amount in a relatively small volume. However, the cryogenic temperature of liquid hydrogen (-253°C) requires the use of specialized insulation and thermal management systems, which can be expensive.
Hydrogen can also be stored in a solid form, by adsorbing it onto the surface of a porous material. This method has the advantage of being relatively simple and safe, and it can achieve a high storage density. However, the rate at which hydrogen can be adsorbed and desorbed from the storage material can be slow, which may limit the practical usefulness of this method in some applications.
Other methods of hydrogen storage, such as chemical storage and metal hydride storage, also exist. These methods involve the use of chemical compounds or metals that can reversibly react with hydrogen to form stable compounds, which can then be stored until they are needed. These methods can achieve high storage densities and are relatively safe, but they may be limited by the rate at which hydrogen can be absorbed and released, as well as by the cost and availability of the storage materials.
Keywords
Compressed hydrogen, pressure tank, liquid hydrogen, crygenic storage tanks, underground containers, gas structures, hydrides of hydrogen, metal hydrides
-
Compressed hydrogen storage refers to the storage of hydrogen gas at high pressures in order to reduce the volume that it occupies. This can be done in a number of different ways, including the use of high-pressure tanks or cylinders.
Compressed hydrogen storage is that it allows for the storage of a relatively large amount of hydrogen in a relatively small space. This makes it an attractive option for use in vehicles and other applications where space is at a premium. However, it is important to note that the high pressures required for compressed hydrogen storage can pose safety risks, and the tanks and cylinders used to store the gas must be carefully designed and maintained to minimize these risks.
Liquid hydrogen storage refers to the storage of hydrogen in its liquid form, rather than as a gas or solid. To store hydrogen in its liquid form, it must be cooled to a temperature of around -253°C, which is well below its boiling point.
Liquid hydrogen storage is that it allows for the storage of a very large amount of hydrogen in a relatively small volume. This makes it an attractive option for use in a variety of applications, including space travel, where weight and volume are critical considerations.
The low temperature required for the storage of liquid hydrogen makes it difficult to handle and transport, and it requires specialized equipment and insulation to prevent heat transfer and evaporation. Additionally, the low temperature of liquid hydrogen can make it prone to embrittlement of certain materials, which can cause problems with tanks and other storage vessels.
It is possible to store hydrogen in underground containers in a mixture with methane or ammonia, which is a process known as "hydrogen blending." This approach can potentially be used to store excess hydrogen that is produced from renewable energy sources, such as wind or solar power, and then blend it with natural gas for use as a fuel.
Hydrogen blending is that it allows hydrogen to be stored and transported using existing infrastructure, such as natural gas pipelines. This can be more cost-effective and logistically simpler than building new infrastructure specifically for hydrogen storage and transport.
One of the main issues is that hydrogen and methane or ammonia have different physical and chemical properties, which can make it difficult to blend them together in a way that is safe and effective. Additionally, hydrogen is more expensive to produce than methane, so the economics of hydrogen blending may not always be favorable.
Hydrogen storage in hydrides refers to the use of materials that can absorb and release large amounts of hydrogen, known as "hydrides," as a way to store hydrogen. There are several types of hydrides that can be used for hydrogen storage, including metal hydrides, chemical hydrides, and complex hydrides.
Main advantages of hydrogen storage in hydrides is that it allows for the storage of hydrogen in a relatively compact and lightweight form. This makes it an attractive option for use in a variety of applications, such as portable electronic devices and fuel cell vehicles.
Hydrides have a relatively low capacity for hydrogen storage, meaning that a large volume of the material is required in order to store a practical amount of hydrogen. Additionally, the process of absorbing and releasing hydrogen from hydrides is often slow and requires the use of heat, which can be energy-intensive and inefficient.
Hydrogen storage in carbon-based containers refers to the use of materials made of carbon, such as carbon nanotubes or graphene, as a way to store hydrogen. These materials are known for their high surface area and strong chemical bonds, which make them capable of adsorbing and storing large amounts of hydrogen.
One of the main advantages of hydrogen storage in carbon-based containers is that they have a high capacity for hydrogen storage, which means that a relatively small volume of the material is required in order to store a practical amount of hydrogen. Additionally, carbon-based materials are relatively lightweight and strong, which makes them suitable for use in a variety of applications.
Review questions
1. Explain the Joule-Thomson effect.
2. What is the typical pressure in high pressure hydrogen storage tanks?
3. What mechanism must tanks be equipped with to store liquid hydrogen?
4. Describe the advantages of storing hydrogen in underground storage mixed with methane or ammonia.
5. What types of hydride are suitable for hydrogen storage.
6. Which carbon materials are suitable for hydrogen storage?
-
-
-
There are several ways to store hydrogen, depending on the type and the desired pressure and temperature conditions. Some common components for storing hydrogen include:
· Compressed hydrogen cylinders: These are high-pressure containers made of steel, aluminum, or composite materials. They are used to store hydrogen that are in a compressed liquid or gaseous state.
· Cryogenic tanks: These tanks are used to store gases that are in a cryogenic (very low temperature) state, such as liquid hydrogen or liquid oxygen.
· High-pressure storage tanks: These are large tanks that are used to store gases at high pressure. They can be made of steel, aluminum, or other materials and are typically used to store hydrogen that are in a gaseous state.
· Pipeline storage: Hydrogen can also be stored in pipelines, which are used to transport the gas from one location to another. These pipelines are typically buried underground and can be used to store gases for long periods of time.
It is important to follow proper safety procedures and regulations when storing hydrogen to minimize the risk of accidents or releases.
Keywords
Compressed cylinders, cryogenic tanks, high-pressure storage tanks, pressure vessels
-
Summary
Compressed hydrogen cylinders are high-pressure containers used to store hydrogen gas at pressures ranging from 350 to 700 bar. These cylinders are made of high-strength materials such as steel or composite materials and are designed to be strong yet lightweight. They are commonly used as a fuel for hydrogen vehicles, as a chemical feedstock, and for other industrial and commercial applications.
Cryogenic hydrogen tanks are storage vessels that are used to store hydrogen gas at extremely low temperatures, typically in the range of -253°C or colder. These tanks are made of materials that can withstand the extreme cold and pressure of the stored hydrogen, such as stainless steel or high-strength composites.
High-pressure hydrogen storage tanks are pressure vessels that are used to store hydrogen gas at high pressures. These tanks are made of high-strength materials such as steel or composite materials and are designed to be strong yet lightweight. They are commonly used as a fuel for hydrogen vehicles, as a chemical feedstock, and for other industrial and commercial applications.
High-pressure hydrogen storage tanks have several advantages over other types of fuel storage. They are relatively lightweight, making them well-suited for use in vehicles. They are also easy to refill and can be used in a wide range of temperatures. However, hydrogen is a highly flammable gas, so it is important to follow proper safety procedures when handling and storing high-pressure hydrogen storage tanks. This includes ensuring that the tanks are stored in a safe location away from sources of ignition and that they are handled and transported carefully to prevent damage.
Review questions
1. What components do you know for hydrogen storage?
2. What is the difference between a pressure vessel and a cryogenic tank?
3. Where is high-pressure hydrogen storage most often used?
-
-