HYDROGEN STORAGE IN CARBON-BASED CONTAINERS
Carbon materials, such as graphene and carbon nanotubes, have been studied as a means of storing hydrogen. These materials have high surface area and can physically absorb hydrogen gas, allowing them to store large amounts of hydrogen in a small volume. However, the hydrogen storage capacity of these materials is limited by their surface area and pore size, and they can only store small amounts of hydrogen at a time. Additionally, the process of absorbing and releasing hydrogen from these materials can be slow, making them less practical for use in hydrogen storage applications.
There are several different ways that hydrogen can be stored in carbon materials, including physical adsorption, chemical adsorption, and chemical reaction.
Physical adsorption occurs when hydrogen molecules are attracted to the surface of the carbon material due to Van der Waals forces. This type of hydrogen storage is reversible, meaning that the hydrogen can be easily released from the material by reducing the pressure or increasing the temperature. Physical adsorption can be used to store hydrogen at high pressures (up to about 35 MPa) and at room temperature, but the storage capacity is limited by the surface area of the material.
Van der Waals forces are weak attractive forces that arise between neutral atoms and molecules. They are a type of non-covalent interaction and are caused by temporary fluctuations in electron density. The three types of van der Waals forces are London dispersion forces, dipole-dipole interactions and quadrupole-quadrupole interactions. These forces are responsible for the behavior of gases and liquids and also play a role in the stability of biomolecules and crystal formation.
Chemical adsorption involves the formation of a chemical bond between the hydrogen and the carbon material. This type of hydrogen storage is also reversible, but the bond is stronger than the van der Waals forces involved in physical adsorption, so it requires more energy to release the hydrogen. Chemical adsorption can be used to store hydrogen at lower pressures (below 10 MPa) and at lower temperatures.
Chemical reaction involves the formation of a new compound by reacting hydrogen with the carbon material. This type of hydrogen storage is not reversible and the hydrogen cannot be easily released from the material. However, chemical reaction can be used to store large amounts of hydrogen in a small volume.
Overall, carbon materials have several attractive properties for hydrogen storage, including high surface area, high strength, and low weight. However, the storage capacity of these materials is limited and the process of absorbing and releasing hydrogen can be slow, making them less practical for use in many hydrogen storage applications.
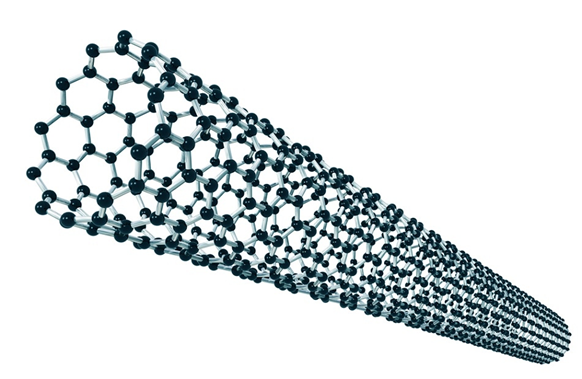
Carbon nanostructures include highly porous graphite and carbon nanotubes. Recently, attention has been focused on the study of single-walled nanotubes, which have great potential for hydrogen storage. Many research teams around the world are working on the issue.
The elementary building element of nanotubes is graphite. Nanotubes are formed by one or several layers coiled into the tube of a final length. The diameter of tubes is 0.7 – 3nm (Krček, 2010, s. 22).