SUMMARY & REVIEW QUESTIONS
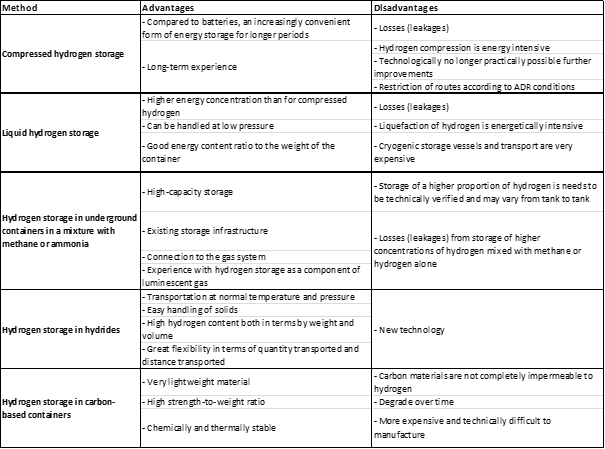
Compressed hydrogen storage refers to the storage of hydrogen gas at high pressures in order to reduce the volume that it occupies. This can be done in a number of different ways, including the use of high-pressure tanks or cylinders.
Compressed hydrogen storage is that it allows for the storage of a relatively large amount of hydrogen in a relatively small space. This makes it an attractive option for use in vehicles and other applications where space is at a premium. However, it is important to note that the high pressures required for compressed hydrogen storage can pose safety risks, and the tanks and cylinders used to store the gas must be carefully designed and maintained to minimize these risks.
Liquid hydrogen storage refers to the storage of hydrogen in its liquid form, rather than as a gas or solid. To store hydrogen in its liquid form, it must be cooled to a temperature of around -253°C, which is well below its boiling point.
Liquid hydrogen storage is that it allows for the storage of a very large amount of hydrogen in a relatively small volume. This makes it an attractive option for use in a variety of applications, including space travel, where weight and volume are critical considerations.
The low temperature required for the storage of liquid hydrogen makes it difficult to handle and transport, and it requires specialized equipment and insulation to prevent heat transfer and evaporation. Additionally, the low temperature of liquid hydrogen can make it prone to embrittlement of certain materials, which can cause problems with tanks and other storage vessels.
It is possible to store hydrogen in underground containers in a mixture with methane or ammonia, which is a process known as "hydrogen blending." This approach can potentially be used to store excess hydrogen that is produced from renewable energy sources, such as wind or solar power, and then blend it with natural gas for use as a fuel.
Hydrogen blending is that it allows hydrogen to be stored and transported using existing infrastructure, such as natural gas pipelines. This can be more cost-effective and logistically simpler than building new infrastructure specifically for hydrogen storage and transport.
One of the main issues is that hydrogen and methane or ammonia have different physical and chemical properties, which can make it difficult to blend them together in a way that is safe and effective. Additionally, hydrogen is more expensive to produce than methane, so the economics of hydrogen blending may not always be favorable.
Hydrogen storage in hydrides refers to the use of materials that can absorb and release large amounts of hydrogen, known as "hydrides," as a way to store hydrogen. There are several types of hydrides that can be used for hydrogen storage, including metal hydrides, chemical hydrides, and complex hydrides.
Main advantages of hydrogen storage in hydrides is that it allows for the storage of hydrogen in a relatively compact and lightweight form. This makes it an attractive option for use in a variety of applications, such as portable electronic devices and fuel cell vehicles.
Hydrides have a relatively low capacity for hydrogen storage, meaning that a large volume of the material is required in order to store a practical amount of hydrogen. Additionally, the process of absorbing and releasing hydrogen from hydrides is often slow and requires the use of heat, which can be energy-intensive and inefficient.
Hydrogen storage in carbon-based containers refers to the use of materials made of carbon, such as carbon nanotubes or graphene, as a way to store hydrogen. These materials are known for their high surface area and strong chemical bonds, which make them capable of adsorbing and storing large amounts of hydrogen.
One of the main advantages of hydrogen storage in carbon-based containers is that they have a high capacity for hydrogen storage, which means that a relatively small volume of the material is required in order to store a practical amount of hydrogen. Additionally, carbon-based materials are relatively lightweight and strong, which makes them suitable for use in a variety of applications.
Review questions
1. Explain the Joule-Thomson effect.
2. What is the typical pressure in high pressure hydrogen storage tanks?
3. What mechanism must tanks be equipped with to store liquid hydrogen?
4. Describe the advantages of storing hydrogen in underground storage mixed with methane or ammonia.
5. What types of hydride are suitable for hydrogen storage.
6. Which carbon materials are suitable for hydrogen storage?
Special | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ALL
C |
|---|