COMPRESSED HYDROGEN STORAGE
Due to the low density of hydrogen it is necessary to store it compressed in pressure tanks. They have to be pressure and destruction resistant and correct tight (tightened with torquewrench) to avoid leaking. Hydrogen is a gas with the smalles molecule and therefore it is necessary to use special materials for its storage. When hydrogen gets in contact with virginal steel or aluminium so called hydrogen embrittlement occurs which can worsen the durability of pressure cylinders which again requires the use of some special materials. Pressing hydrogen itself is energetically demanding. Hydrogen is a very badly compressable gas. It has reversed the Joule-Thompson coefficient, therefore much more energy is needed on its compression than with other gases. For stationary storage of hydrogen there are used large quantity steel pressure tanks or composite (Vodíková strategie České republiky, 2021, s. 81).
The Joule-Thomson effect, also known as the Joule-Kelvin effect, is the temperature change of a gas or liquid as it expands or is forced through a small opening or porous material. This effect is named after James Joule and William Thomson (Lord Kelvin).
The Joule-Thomson effect can be observed when a high-pressure gas is allowed to expand through a small opening or porous material. As the gas expands, its temperature decreases. This is because the expansion of the gas does work on the surroundings, which requires energy. The energy needed for this work is taken from the internal energy of the gas, causing its temperature to drop.
The Joule-Thomson coefficient is a measure of the temperature change of a gas or liquid as it expands. The coefficient is positive for most gases, which means that the temperature of the gas decreases as it expands. For liquids, the Joule-Thomson coefficient can be either positive or negative, depending on the specific properties of the liquid.
The Joule-Thomson effect has practical applications in refrigeration and air conditioning, as well as in the oil and gas industry, where it is used to measure the temperature and pressure of natural gas at various stages in the production process.
For stationary application steel weldless cylinders from low-carbon or alloyed steel are usually used. They are made at volumes ranging from several litres up to approximately 50 l for common application. For mobile applications there are usually used some composite pressure containers. They are made at volumes from tens of litres up to approximately 300 l. A typical operational pressure is 350 bar, in the newest applications it is 450 – 700 bar. In many applications, the cylindrical shape is slightly deformed depending on the needs for installation into storage space of a vehicle. If one wants to store hydrogen in high pressure tanks, first, it has to be compressed at a required pressure. For compressing hydrogen piston compressors are mainly used. The energy necessary for hydrogen compression at 350 bar reaches approximately 30% of energy from a fuel (Krátky, 2012, s. 37).

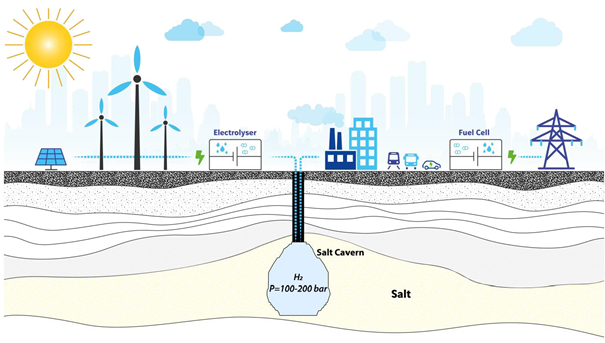
Next option of hydrogen storage in a gaseous form is to store it in underground storage sites. They are usually some extracted salt mines or caves of natural gas and empty gas field. In the world this method is used on several places, for example in Amarillo in Texas (850 mil. m3), in French Beynes (330 mil. m3), in English Billington (2.2 mil. m3). Other storage sites can be found, for example, in Germany and Holland (Krček, 2010, s. 20).