WATER ELECTROLYSIS
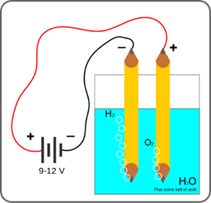
During water electrolysis, water is separated into hydrogen and oxygen. Direct electric current flows through the aqueous solution and splits chemical bonds between hydrogen and oxygen. Water reacts at the anode to form O2 and at the cathode H2 (F. 5) 2H2O ⟶ 2 H2 + O2.
Hydrogen produced at the cathode is collected and stored. The process can be done at room temperature, and only electricity is needed. This method produces highly pure hydrogen gas with no need to be further purification. Consequently, this method is suitable where pure hydrogen and oxygen are needed. The apparatus used for electrolysis is called an electrolyser. It consists of a container, electrode, and electrolyte.
The efficiency of the process is somewhere between 80 – 92%, and it can be increased by the additional electrolyte, which boosts water conductivity. Electrolysis is not used mainly because of the high cost of electricity. The efficiency of electric power production influences the efficiency of electrolysis. Currently, the efficacy of electric energy production it is between 30-40% using available resources; therefore, the efficacy of electrolysis is somewhere between 25-35%.
Compared to other methods, electrolysis has a high energy demand. Power consumption is 5.2 kWh per 1m3 of hydrogen which makes it 57 kWh per 1 kg. Electrolysis is a promising option for carbon-free hydrogen production from renewable resources.[1]
Electrolysis is used where affordable "green" energy is available and where there is a surplus of energy. In addition, oxygen is produced which can also be used.
[1] DOUCEK, A., JANÍK, L., TENKRÁT, D., DLOUHÝ, P. Využití vodíku k regulaci obnovitelných zdrojů energie [online]. Chemagazín, 2010, č.3, roč. 20. Available at: http://www.chemagazin.cz/userdata/chemagazin_2010/ file/CHXX3_cl1.pdf