PRINCIPLE
Fuel cells are generally defined as electrochemical devices and their function is to convert hydrogen (fuel) and oxygen (oxidizer) into electrical energy. These galvanic cells contain two electrodes separated by a membrane or an electrolyte. The fuel (hydrogen) is supplied to the positive electrode, while the oxidizing agent (oxygen) is supplied to the negative electrode. Electrons are created at the positive electrode (anode)and flow through an external electrical circuit to the negative electrode (cathode), generating an electric current. In theory, a fuel cell can operate continuously as long the supply of fuel or oxidizer to the electrodes is not interrupted.
There are many combinations of fuel and oxidizer. For example, an oxy-hydrogen cell uses hydrogen as fuel and oxygen as an oxidizer, producing pure water as a waste product. Other cells use hydrocarbons and alcohols as fuels. Instead of pure oxygen, air, chlorine, or chlorine dioxide can be used as oxidizing agents.
Electrodes can be made of carbon (nanotubes) or various metals, and their efficiency can be increased by coating them with catalysts, such as palladium or platinum.
Different acids, mostly phosphoric acid (H3PO4), or bases, most commonly potassium hydroxide (KOH), ceramics, or membranes can serve as electrolyte. In specific fuel cells, gas under high pressure is used as the electrolyte. The most widely used electrolyte today is KOH, which was already used in cells in the Apollo project. However, the disadvantage of this electrolyte is that the oxidizer must be cleaned of CO2 to prevent carbon dioxide from reacting with it, as the resulting potassium carbonate would cease to fulfil the function of the electrolyte.
The resulting electrical voltage is theoretically around 1.23 volts, and its value depends on the type of fuel used and the quality of the cell. Currently, most commonly used cells typically produce a voltage of 0.5 - 0.95 V. To achieve a higher voltage, multiple fuel cells can be connected in series. The magnitude of the current depends on the surface area of the cell, and commercially available cells today can provide approximately 0.5W/cm².
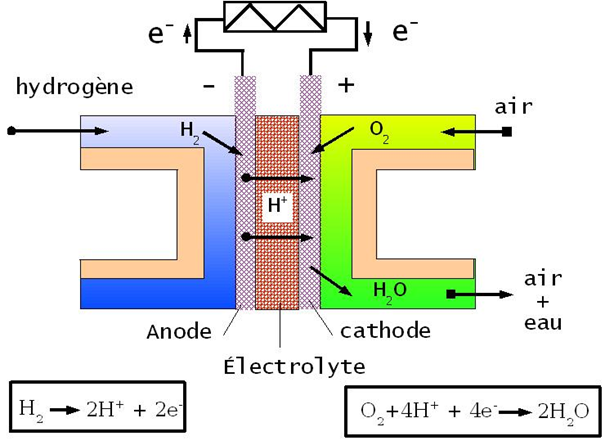
The fuel is catalytically oxidized at the anode to produce cations (such as hydrogen to H+). These cations pass through the membrane or into the electrolyte. The released electrons are collected at the anode and travel towards the electrical device. Because electrons carry a negative charge, the electric current flows in the opposite direction, from the cathode (+), through the electrical device to the anode (−). At the cathode, the oxidizing agent is reduced to anions (such as oxygen to O2−), which then combine with cations (for example, hydrogen and oxygen to form water).