STEAM REFORMATION
During the steam reformation, hydrocarbon (e.g., methane) reacts with water steam in the catalyst. The product of this process is carbon monoxide, hydrogen [1] and in the residual steam, carbon dioxide [2]. If the source used contains a sulphur compound, desulphurisation is necessary.
[1] CH4 + H2O ⟶ CO + 3H2
[2] CH4 + 2H2O ⟶ CO2 + 4H2
The pressure of 3-5 MPa and temperatures between 750 – 800 °C are applied. Nickel oxide is used as a catalyst. The ratio of steam is 3:1 to avoid carbon settlement in the catalyst. 9 Produced Carbon monoxide undergoes a water-gas shift, and more carbon dioxide and hydrogen are manufactured. This reaction is exothermic and is implemented in two stages. During the first stage, iron oxide and chromium oxide are used as catalysts. It is a less reactive catalyst and is resistant to impurities. The reactor entry temperature is 380°C, and the exit temperature is 500°C. During the second stage of the process, much lower temperatures are used (180 – 230°C). This is allowed by using a highly reactive copper catalyst. This way, the concentration of carbon monoxide is decreased to 0.2 – 0.3%.
[3] CO + H2O ⟶ CO2 + H2
Hydrogen used for hydrogenation cannot contain oxygen compounds (CO and CO2) and must be converted back into methane [4,5]. This process is done in a methanation reactor at a temperature of around 400°C. If the amount of CO and CO2 in raw gas exceeds 3%, it is necessary to cool it down as both reactions are exothermic.
[4] CO + 3H2 ⟶ CH4 + H2O
[5] CO2 + 4H2 ⟶ CH4 + 2H2O
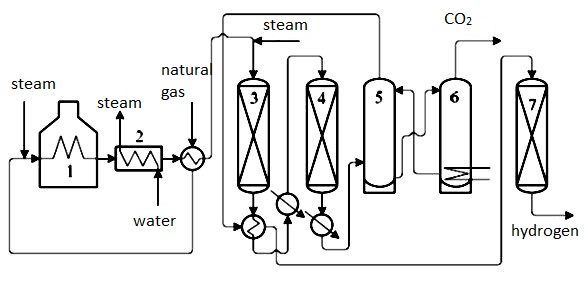
Figure number one shows a simplified diagram of Steam reformation using natural gas. Heated natural gas, after desulphurisation, is mixed with steam in a reformer where reactions [1] and [2] occur. First, products go through a reformer heated to 750°C and then to a shift reactor where they are cooled to 360°C. The following two stages are high-temperature and low-temperature shift converters, where CO is converted to CO2 [3]. Gases are then led to the absorber, where using ethanolamine or other means, CO2 is absorbed. Finally, residual CO and CO2 are converted to methane in a methanation reactor [4,5]. This way, hydrogen of 98% purity is produced, and the remaining 2% is mostly methane. The effectivity of steam reformation is between 70 – 85% depending on hydrogen purity and the amount of steam to carbon ratio (Figure 2). Carbon dioxide produced during steam reforming or partial oxidation is released into the air, purified, liquidated, or turned into solid (dry ice) and used for cooling in the food industry.