PARTIAL OXIDATION
Partial oxidation is quite common in hydrogen production: gas and liquid materials from primary and secondary oil processing are used. Frequently, heavy oil residue fractions are gasified (Vacuum residuals, propane asphalt and others). The gasification process uses oxygen and steam at 1300-1500 C and 3-8 MPa. A limited amount of oxygen allows partial oxidation. The partial oxidation of hydrocarbon sources (CnHm) uses partly reactions [6] and partly [7]. The product of the reaction is carbon dioxide, carbon monoxide and hydrogen. Both reactions are exothermic and heat the mixture to 1500 C.
[6] 2CnHm + nO2 ⟶ 2nCO + mH2
[7] CnHm + nO2 ⟶ nCO2 + m/2H2
The part of the sources not gasified by oxidation is gasified by endothermic reaction using steam [8]. Steam gasification leads to high hydrogen profit and to lowering process temperatures to 1350 C.
[8] CnHm + nH2O ⟶ nCO + n+m/2H2
The product of partial oxidation of different sources is always a mixture of CO, CO2, H2O, H2, CH4 and sulphuric compounds H2S and COS. The harmful byproduct is soot.
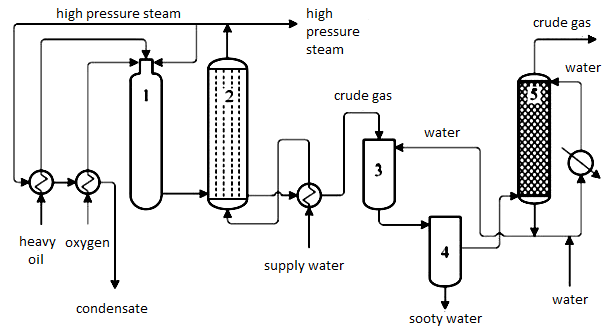
Figure 3 shows a simplified diagram of the partial oxidation of heavy oil residue. Warmed heavy oil residue is dispersed into a stream of steam and oxygen mixture. In a generator, the gas of 1350 C is produced and connected to a steam reactor. The gas is rapidly moved through the reactor to avoid soot sediment. The gas is then cooled in the boiler above the temperature of saturated water steam (around 260 C), and at the same time, high-pressure steam of 12 MPa is produced. Some steam is used in the partial oxidation process (20%), and the rest is in other applications. In the next part of the process, generator gas is cooled by water dispersion in a radiator which removes part of the soot. Finally, the gas is completely purified in a gas purifier.
Sulphane is removed from the final generator gas. The CO is converted to CO2, which is also removed from the gas. Residual CO and CO2 are then extracted using methanation. Conversion, purification and methanation are processed in the same manner as with natural gas.
The efficacy of partial oxidation of oil fractions is generally lower than steam reformation, usually around 50%. As with steam reformation, the investment demands are very high, but these are not included in my energetic needs compared to 1 m3 of hydrogen. Partial oxidation needs higher pressure and temperature than steam reformation; therefore, the energetic demands are higher. When considering the environment, partial oxidation is not better than steam reformation. A large amount of greenhouse gasses is produced. According to the limited use of heavy oil residue, few selling options and dwindling supplies of fossil fuels, partial oxidation has a higher potential for its use